Stem Cell Research
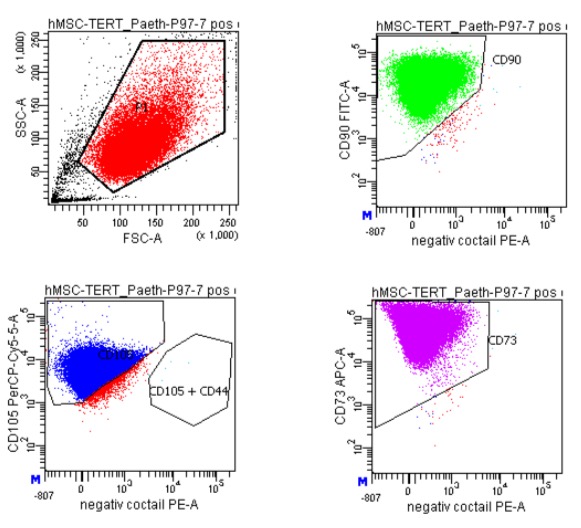
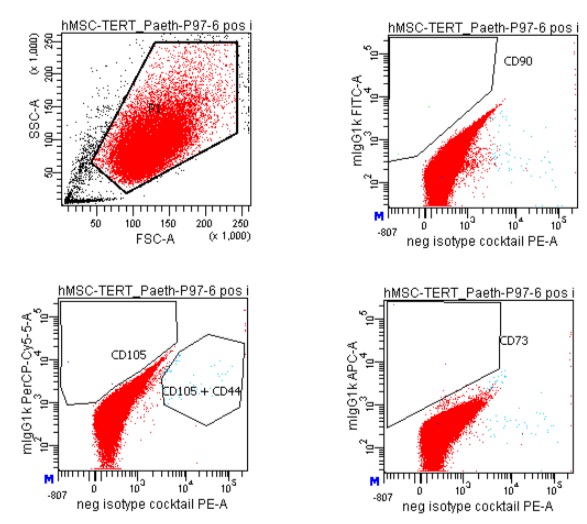
FACS of hBMMSC-tert: Disassociated, washed cells were stained with different fluorescent dye mixtures. After compensation and gating cells, stained with the positive marker (CD105, CD73, CD90,) and the negative marker cocktail (CD45, CD34, CD11b, CD19, HLA-DR) were measured with a BD FACS Canto (left figure). The right figure shows cells stained with the isotype controls of the positive cocktail. (negative marker cocktails not shown).
Stem cell Research
Fetal pancreas development. In order to make use of stem cells either as a source of pancreatic beta cell-like cells or as supply cells to support survival of pancreatic islets after pancreatic islet transplantation is helpful to understand pancreas development. The latter is guided by changes in transcription factor networks conveyed by external and internal signal inputs. For instance, Pdx1 is considered one master regulator of pancreatic development and differentiation into beta cell. Agenesis of pancreas is seen in mice with the targeted disruption of the Pdx1 gene and also seen in humans associated with mutations in human homologue. The pattern of Pdx1 expression is preserved throughout development providing spatial and temporal contributions to the commitment of endoderm to a pancreatic phenotype. Pdx1 expression becomes mostly restricted to beta cells and with lower expression seen in somatostatin and polypeptide producing cells. It also plays a role in beta cells’ terminal differentiation by inducing expression of insulin, glucose transporter 2, glucokinase and islet amyloid polypeptide.
Further specification of endocrine progenitors is required. This is regulated by a basic helix-loop-helix family transcription factor neurogenin 3 (Ngn3) activity. Ngn3 expression is essential for the development of all islet cells. Ngn3 further activates Beta2/NeuroD1, which is confined to mature islets and contributes to endocrine survival as well as insulin gene transcription.
Additional transcription factors are required for further specification and differentiation of islet cells, including Arx, Pax6, Pax4, Nkx2.2, Nkx6.1, Isl1, MafA, and MafB. Isl1, which is a LIM-Homeodomain transcription factor, initially identified as an insulin gene enhancer binding protein is necessary for the survival, maturation and proliferation of the endocrine pancreas. Loss of Isl-1 from the mouse pancreatic epithelium at E13.5 day leads to a severe reduction in hormone- expressing cells and the eventual loss of islet mass. MafA a basic-leucine zipper transcription factor has been identified as an important regulator of beta cell function and a marker for mature beta cells. It has been shown that insulin gene is a direct transcriptional target of MafA In contrast Arx is a marker for the islet alpha-cell lineage and plays an essential role in the alpha-cell development.
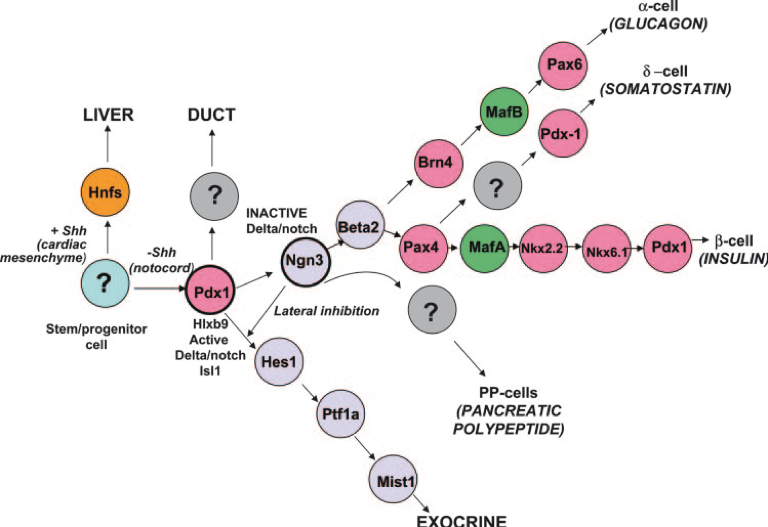
Hierarchy of transcription factor expression in the developing pancreas in the mouse by Naga Deepa Kandula adapted from Habener et al., 2005, Endocrinology
Why adult stem cells ? Mesenchymal stem cells (MSC) are non-hematopoietic, fibroblast like, multipotent stromal cells from bone marrow and connective tissue that transform into osteoblast, chondroblast and adipocyte lineages. As they have recently been reported to differentiate into endoderm-like cells, they are considered an interesting source of oligopotent stem cells with less ethical issues as they can be taken from adult donor as opposed to pluripotent stem cells of fetal origin. MSC can secrete angiogenic and trophic factors as well as having immunomodulatory properties,which may protect residual pancreatic islets from damage or improve engraftment of transplanted islets. Isolation of adipose-tissue derived MSC (AdMSC) could provide another rich source,which can be utilized for therapeutic purposes.
Human MSC are considered genetically stable but could undergo malignant transformation. This condition is not acceptable and should be ruled out for therapeutic use. Efficacy of MSC transfer from different routes should be exploited for better protection of islets. In this project we investigate the capacity of human MSC to support regeneration of pancreatic islets damaged by multiple dosages of streptozotocin or partial pancreatectomy in NMRI nu/nu mice.
OBJECTIVES
Find out if pancreatic progenitor cells can be generated from MSC in cell culture conditions.
Effect of nutrients, such as fatty acids and glucose on MSC differentiation and proliferation properties in culture.
Improvement of diabetes and regeneration of the endocrine pancreas by MSC injection in diabetic mice.